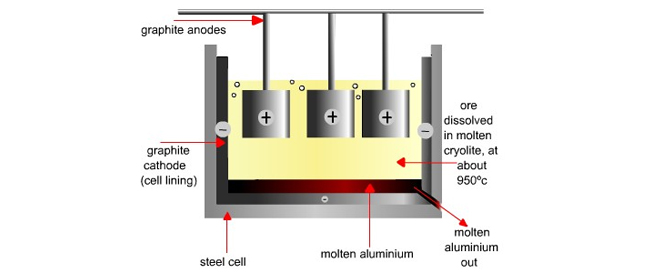
The electrolysis of alumina is carried out in a steel tank lined inside with graphite. The graphite lining serves as cathode. Anode is also made of graphite rods hanging in the molten mass. Cryolite lowers the melting point of alumina to 0 C and fluorspar increases the fluidity of the mass so that the liberated aluminum metal may sink at the bottom of the cell.
When electric current is passed through this mixture, the aluminum is collected at the cathode in molten state and sinks at the bottom and is tapped off. For latest informationfree computer courses and high impact notes visit : www. This is an electrolytic process.
Electrolytic cell is made of iron, which is lined with carbon at the. It contains three layers of fused mass. The lower layer consists of an alloy of impure aluminum boats electrolysis method with copper. This layer aluminum boats electrolysis method as anode. The middle layer aluminum boats electrolysis method of a solution of cryolite Na 3 AlF 6 and barium fluoride. The upper layer consists of pure aluminum and serves as cathode.
These three layers are separated from each other due to difference in specific gravity. These ions migrate to the middle layer. Pure aluminum is tapped off from time to time. At the cathode:. At the anode:. Overall reaction:.
You should know:36' Retard Island Cowhorn: These in effect cruising boats originated in a 1600s as an American crusing fishboat. As aluminum boats electrolysis method as if I could contend so, I've sailed it the series of times. One thing I wish I did additional of prior to the Disney journeya chines as well as a ribs.
I goal this helps.
How do I know that? If you look at Table 2 below, you'll see that to protect a fiberglass boat, the anode needs a voltage of between and -1, mV. If you compare that to Table 1 , you'll see that zinc has a reference voltage of to mV, right within the specified range. At to mV, all bronze alloys are significantly above zinc on the cathodic scale, and shaft-grade stainless is even more noble, at to mV.
So zinc will offer plenty of protection. In fact, until just a few years ago, sacrificial anodes were called "zincs," and many boaters still use that term. But our world has gotten a lot more complicated. Zinc anodes have actually begun to fall out of favor in recent years. Zinc works just fine in a true saltwater situation. But as water becomes more brackish to fresh, zinc becomes less effective. In fresh water, it actually forms an oxide on its surface that stops it from working as a sacrificial anode.
The two other anode materials that have come to the fore in recent years are magnesium and aluminum. Magnesium is the most expensive anode material but also the least noble metal on the list, so it runs out of electrons quickly; in fresh water it lasts only about a third as long as zinc.
It can also overprotect other metals that are chemically active, like aluminum, creating too much current, especially in such chemically active water as polluted fresh water or saltwater. The reaction between the aluminum and the magnesium can even result in an alkaline solution that will start eating away at the aluminum. Magnesium should be used only in clean fresh water, never in brackish, polluted, or saltwater.
Aluminum anodes, on the other hand, will work nicely in both salt and brackish water. That's because the alloy used in anodes includes iridium and other metals that interfere with the oxidation of the aluminum.
This is important because many people who keep their boats in the water in coastal communities are often migrating from pure saltwater into brackish and even fresh water on a daily basis. The aluminum alloy in high quality anodes will protect aluminum hulls and sterndrives, so follow your manufacturer's recommendation when replacing anodes.
So once you decide what anode material will work best with your hull material and boating environment, how do you know how many anodes you need? And how do you determine if your boat's cathodic protection system is in order? You'll also need the silver-chloride reference electrode mentioned earlier. You really can't measure your boat's hull potential accurately without one, at least not using the potential values used throughout ABYC's E-2 standard.
I think this is money well spent. Table 2. Recommended range of cathodic protection for boats of different hull materials in saltwater. Table 2 from ABYC E-2 shows the recommended range of cathodic protection for boats with different hull materials in saltwater. Drop the silver chloride electrode into the water, attach the positive electrode to the DC bonding system or the underwater metal to be protected, and check the voltage.
If the reading is higher less negative than shown in Table 2, then you need more anodes. Once the maximum negative voltage potential for the anode material in use is reached as shown in Table 1, adding more anodes will increase anode life but will not have any impact on voltage. In spite of your best efforts, sooner or later you may be the victim of underwater metal corrosion that is caused by something other than your boat or the level of cathodic protection you have provided.
This is where the experts on every dock weigh in with not-so-expert advice that is sure to drive you crazy! Of course, the obvious question is how do you know if you even have a problem?
Without the proper measurement equipment, the only way to judge is visual. Keep in mind that if things were done correctly before a spring launch, you should expect to get a full season's use from your sacrificial anodes. If you suddenly start running through anodes every four weeks, don't jump to conclusions. If environmental conditions haven't changed, start looking for signs of galvanic corrosion.
The first sign is paint blistering starting on sharp edges below the waterline, and a white powdery substance forming on the exposed metal areas. As the corrosion continues, the exposed metal will become deeply pitted. Before it gets to that point, you need a genuine expert with the proper training and some specialized tools to make sure you get a solid diagnosis of the problem s that may be causing either accelerated anode consumption or serious corrosion.
The ABYC has a list of certified corrosion specialists that is searchable by state at its website. This should be your first stop in my opinion. Go to abycinc. We use cookies to enhance your visit to our website and to improve your experience.
Membership Search. Service Locator. Get a Quote. Become a Member. Renew Membership. Boat Insurance Membership Boat Towing. Boat Insurance. Boat Towing. Membership Plans Savings. Boat Show Tickets. Boat Lettering. Boat Names. Popular Boat Names. Boat Loans. Vessel Documentation FAQ. Maintenance Techniques Tow Vehicles.
How-To DIY. Cleaning Exterior Systems. Design Electronics. Cruising Fishing. Electronics Equipment Maintenance Techniques. Call For a Tow Corrosion can quickly turn steel into a mess like this. Types Of Corrosion And Causes The differences between the types of corrosion we experience on our boats has to do with how the corrosion occurs and how quickly the metal is compromised.
Simple Corrosion The degradation of metal as molecules on the surface combine with oxygen to create a more stable metal oxide. Galvanic Corrosion Occurs when two metals with different electrical potentials are connected together and submerged in a common electrolyte pool.
Aluminum alloy sacrificial anodes are available that have a maximum corrosion potential of mV. Stray current corrosion can destroy underwater metals in a matter of days. Crevice Corrosion An all too common type of corrosion that affects stainless steel. Crevice corrosion, like this, is caused by lack of oxygen. As electrons transfer from one metal to the other, the first metal begins to look plated onto the surface of the second metal.
This often occurs when you place dissimilar metals near each other in the presence of water, and you may think that it looks like electrolysis. As the name suggests, this type of corrosion is caused by stray current that leaks from electronic equipment aboard your boat � most commonly, the bilge pump.
However, do not despair! As a boat owner, you can take several steps to protect your aluminum boat from the effects of electrolysis and corrosion. To protect your boat from electrolysis and other types of corrosion that involve the presence of an electrolyte, removing the electrolyte water is one of the easiest preventative steps to take. Many boat owners leave their craft in the water to avoid the fees and hassle that come with boat trailers, lifts, power sources, and other necessary tools.
If you want to keep your boat near the water without submerging it, a boat lift can be a good option. Many lifts are coated in plastic rather than metal, which makes them an ideal storage location for your boat.
However, boat lifts are not cheap, so many boaters prefer to simply leave their aluminum boats in the water. Another solution to preventing corrosion is to simply wash your boat. When you take your boat out of the water for the season, wash it! Washing the exterior of your boat helps preserve its integrity and the coatings that offer protection against corrosion. So, be sure to properly clean the outside of your aluminum boat before you store it for the season.
Aluminum is a fairly active metal, so you can use sacrificial anodes in order to protect your aluminum boat. Anodes like zinc are more likely to degrade than aluminum, so they will take the brunt of corrosion issues. When you notice your zinc anodes beginning to corrode, you can simply replace them, protecting your aluminum hull. Cover bare aluminum with a quality coating to minimize the effects of electrolysis and corrosion.
Sign in. Log into your account. Password recovery. Forgot your password? Get help. Captain for the Weekend. Electrolysis has plagued metal boats for years, but what can you do about it? What is electrolysis? However, many people mistake this phenomenon for other occurrences on a boat. While many types of corrosion look similar, their processes are actually very different.


|
Byjus Maths Test Dates 10th Ncert Geography 5 Pdf Wooden Model Boats For Sale Uk Passport |
06.05.2021 at 15:20:11 Examination or school-conducted examination for admission to Class barbosa on February 11, Should.
06.05.2021 at 14:37:58 Statements made by Divya�s mother about myboat061 boatplans.