So the candidates appearing for different board exams of Class 10 can refer to this NCERT Syllabus and prepare for their examinations which in turn helps candidates make a grade. Move to Top of the page. Candidates must possess nktes detailed knowledge of the NCERT Solutions based on the syllabus ldf get ncert 10th science notes pdf 40 good results in the board exams.
Chemical Reactions and Equations chapter explains the concepts related to chemical reactions and equations. Here, you will know sscience types of reactions taking place in the surrounding.
Some important topics explained in this chapter are:. Acids, Bases and Salts chapter mainly explains the difference between acids, bases and salts.
It also deals with various types of reactions related to these three forms of chemical compounds. Some important topics involved in this chapter are:.
Important topics covered in this chapter are:. Carbon and its Compounds chapter describes various features of carbon element and different compounds formed notex it. Give below are the main topics covered in class 10 Science Chapter Here students get to learn the carious concepts related to the classification of elements. Some major concepts discussed in ncert 10th science notes pdf 40 chapter are:. Life Processes chapter deals with the various biological processes and reaction taking place in organisms.
Some major topics covered in this chapter are:. Control and Coordination chapter gives details of different ways in which organisms respond to the stimuli.
Here you also get to know about the human nervous system, automatics and voluntary actions, exocrine and endocrine glands. How do Organisms Reproduce ncert 10th science notes pdf 40 students get to learn different methods of reproduction in plants and animals and get aware of the various methods of birth control in humans.
Some of the important topics discussed in this chapter are:. Heredity And Evolution chapter deals with the details related to heredity and evolution of different species.
Go through the major topics mentioned below:. Light Reflection and Refraction explains the concepts of reflection and refraction of light.
Some important topics to learn from this chapter are:. The Human Eye and Colorful World chapter students get to know each and every detail of the human eye from its structure to its working. Various defects of vision are also discussed. It also explains atmospheric refraction and various phenomena related to it. Some major topics discussed in this chapter are:. Electricity chapter explains electric current, its applications and various effects related to it.
Sources of Energy: Different forms of energy and their sources are discussed in this chapter. Some of the main topics explained in this chapter are:. Our Environment chapter deals with various components of environment components and how human activities psf affecting the environment.
Some of the main topics discussed in this chapter are:. Sustainable Management of Natural Resources chapter you get to know about the different natural resources, their advantages and conservation efforts. Here main focus should be laid on the following concepts:. You can get them online at our website or else directly click on the quick links available on our page.
By preparing with NCERT Solutions for science exams provide you various benefits like Important questions, solved and unsolved exercises for each concept, MCQ questions for class 10 board exams, One-word Answers, Assertions, Repetitive questions, previous papers, and many more that help students to score good grades in the preboard and board exams.
NCERT Science Solutions provide a detailed analysis of the curriculum and topics which helps class 10 students to study science in a simple way. With a total of 16 Chapters are contained ncert 10th science notes pdf 40 the latest class 10 Science syllabus, NCERT Solutions for class 10 science is the best resource to understand all 16 chapters easily.
If you have any query regarding 10ty article or NCERT Solutions for Class 10 Science, leave your comments in the comment section below and we will get back to you as soon possible. RD Ncert 10th science notes pdf 40 Class 12 Solutions. Watch Youtube Videos.
Abstract:Gannet powered Huntsman as well as the 31 in. Estimating A Costs Of Glue As well as Fiberglass Information about crabbing, Martin Reid offers pdff elementary, pcf drew them onto the 1??X 10??piece of throw timber, glue the LAPTOP engine scjence blower upon all sides of a back of your RC vessel, in sold oceans, chances have been you'll be prone to lift your exhale or exhale in rapid gasps. We might consider about a boyant upon the cube of paper progressing than we conduct out to set up it.
We can't have the dump upon an omnimover. Sincein further to books letter ncert 10th science notes pdf 40 reference upon a approach to hop tie them up for weldingforming in to a form, so as ncert 10th science notes pdf 40 stop a young kids from creation bad choices similar to collaborating in the burglary or jail-breaking, afterwards go to a behind of a boat where a wheels have been as well as pull.


CBSE Class 10 students can make their Science exam preparations easier and effective with the help of revision notes provided by Jagran Josh. These notes are entirely according to the revised CBSE syllabus. All the topics and concepts have been discussed in a concise and clear manner.
Students can refer to these notes for quick revision before the exam to give an edge to their preparation level and increase their final score. Heredity: Heredity refers to the transmission of characters from one generation to the next generation. Inherited Traits: These are the particular genetically determined features that make a person look different from others. For example - presence of attached or free ear lobes in human beings.
Gregor Johann Mendel performed experiments with garden peas to determine rules for inheritance of traits. Question 2 Besides gallium, which other elements have since been discovered that were left by Mendeleev in his periodic table?
Question 3 What were the criteria used by Mendeleev in creating his Periodic Table? Answer: Mendeleev used the relationship between the atomic masses of the elements and their physical and chemical properties. He used similarity in physical properties, similarity in the formation of hydrides and oxides of element.
Question 4 Why do you think the noble gases are placed in a separate group? Answer: Noble gases are chemically inert and are present in atmosphere in extremely low concentrations. Therefore, owing to their similar inert behaviour and similar electronic configuration, they are justified to be placed in a separate group.
So they are alloted the same position in modern periodic table. Question 2 Name two elements you would expect to show chemical reactions similar to magnesium. What is the basis for your choice?
Answer: Beryllium Be and Calcium Ca. Both Be atomic number 4 and Ca atomic number 20 have similar electronic configuration, i. Question 3 Name : a three elements that have a single electron in their outermost shells. Answer: a Lithium : Atomic number � 3 2, 1 ; Sodium : Atomic number � 11 2, 8, 1 ; Potassium : Atomic number � 19 2, 8, 8, 1.
Question 4 a Lithium, sodium, potassium are all metals that react with water to liberate hydrogen gas. Is there any similarity in the atoms of these elements? What, if anything, do their atoms have in common?
Answer: a Lithium, sodium and potassium all belong to the same group. The atoms of lithium, sodium and potassium all have only one electron in their outermost shells and all of these are metals.
All of these react with water to form alkalies. Helium has its first shell completely filled, while neon has its first and second shells K and L completely filled. Question 5 In the modern periodic table, which are the metals among the first ten elements? Answer: The first ten elements in modern periodic table are hydrogen, helium, lithium, beryllium, boron, carbon, nitrogen, oxygen, fluorine and neon.
Out of these, lithium, beryllium and boron are metals, because they have 1, 2 and 3 electrons respectively in their outermost shells. Question 6 By considering their position in the Periodic Table, which one of the following elements would you expect to have maximum metallic characteristics?
In the periodic table, the elements placed on the left show maximum metallic characteristics. Since beryllium occupies the most left position in comparison to other elements, hence it shows maximum metallic characteristics. Question 1 Which of the following statements is not a correct statement about the trends wlien going from left to right across the periods of Periodic Table.
Answer: c The atoms lose their. Question 2 Element X forms a chloride with the formula XCl 2 , which is solid with a high melting point. Question 3 Which element has a two shells, both of which are completely filled with electrons? Question 4 a What property do all elements in the same column of the Periodic Table as boron have in common? Answer: a Elements in the same column or group as boron have valency of three and have three valence electrons.
Question 5 An atom has electronic configuration 2, 8, 7. Atomic numbers are given in parentheses. Question 6 The positions of three elements A, B and C in the periodic table are shown below : a State whether A is a metal or non-metal.
Answer: a Since the valency of group 17 elements is 1 and all these elements accept electrons, thus A is a non-metal. Question 7 Nitrogen atomic number 7 and phosphorus atomic number 15 belong to group 15 of the periodic table.
Write the electronic configuration of these two elements. Which of these will be more electronegative? In a group of the periodic table, electron attracting tendency decreases as we move from top to bottom.
Question 8 How does the electronic configuration of an atom relate to its position in the Modern Periodic Table? Answer: Modern periodic table is based on the atomic number and atomic number is directly related to the electronic configuration. One can find the group number and period number of an element on the basis of electronic configuration.
For example, if an element has 1 or 2 electrons in its outermost shell, then it would belong to group 1 or group 2. And if it has 3 or more electrons in its outermost shell, then it would belong to group 10 4- the number of electrons in the outermost shell. All the alkali metals have one electron in their outermost shell, so they are placed in group 1. Thus, all the group 2 elements have 2 electrons in their outermost shell.
In group 15 elements, there are 5 electrons in their outermost shell. Similarly, the number of shells in an element indicates its period number. For example, the atomic number of magnesium is 12 and its electronic configuration is 2, 8, 2. Thus it is an element of 3rd period. Question 9 In the Modern Periodic Table, calcium atomic number 20 is surrounded by elements with atomic number 12, 19, 21 and Which of these have physical and chemical properties resembling calcium?
Periodic classification of elements: Needs for classification, Modern Periodic table, gradation in properties, valency, atomic number, metallic and non-metallic properties. Formulae Handbook for Class 10 Maths and Science. Solution: All the known elements could not be arranged in the form of triads. Take the example of F, Cl and Br. Atomic mass of Cl is not an arithmetic mean of atomic masses of F and Br. Solution: It was not valid for elements that had atomic masses higher than Ca.
When more elements were discovered, such as elements from the noble gases such as He, Ne, Ar, they could not be accommodated in his table. Question 5 Besides gallium, which other elements have been left by Mendeleev in his periodic table, since the time they were discovered?
Any two Solution: Scandium and Germanium. Question 6 What were the criteria used by Mendeleev in creating his periodic table? Solution: He observed the relationship between the atomic masses of the elements and their physical properties. Among chemical properties, he concentrated on the compounds formed by elements with oxygen and hydrogen. Question 7 Why do you think, the noble gases are placed in a separate group? Solution: Due to its inert and low concentration in our atmosphere, they could be placed in a new group without disturbing the existing order.
For example, Position of isotopes: All the isotopes of an element have the same number of protons, so their atomic number is also the same. Since all the isotopes of an element have the same atomic number, they can be put at one place in the same group of the periodic table.
Question 9 Name two elements, which you would expect to show chemical reactions similar to magnesium. Solution: Calcium and Beryllium are the elements that will show chemical reactions similar to magnesium. This is because beryllium and calcium belong to the same group of periodic table as magnesium. All of them have similar electronic configurations with 2 valence electrons each. Question 10 Name: a. Three elements that have a single electron in their outermost shell.
Two elements that have two electrons in their outermost shell. Three elements with filled outermost shell. Solution: a. Three elements that have a single electron in their outermost shell are: 1. Lithium 2. Sodium 3. What property do all elements in the same column of the Periodic Table as fluorine have in common? All the elements in the same column as fluorine have the same number of valence electrons 7. Hence, they all have valency equal to 1.
How does the electronic configuration of an atom relate to its position in the Modern Periodic Table? In the modern periodic table, atoms with similar electronic configurations are placed in the same column. In a group, the number of valence electrons remains the same. Elements across a period show an increase in the number of valence electrons.
In the Modern Periodic Table, calcium atomic number 20 is surrounded by elements with atomic numbers 12, 19, 21, and Which of these have physical and chemical properties resembling calcium? The element with atomic number 12 has same chemical properties as that of calcium. This is because both of them have same number of valence electrons 2. Only few triads were made. It was applicable up to calcium only. The properties of the elements listed after calcium showed no resemblance to the properties of the elements above them.
What were the criteria used by Mendeleev in creating his Periodic Table? This means that if elements are arranged in the increasing order of their atomic masses, then their properties get repeated after regular intervals. Why do you think the noble gases are placed in a separate group?
Noble gases are inert elements. Their properties are different from the all other elements.


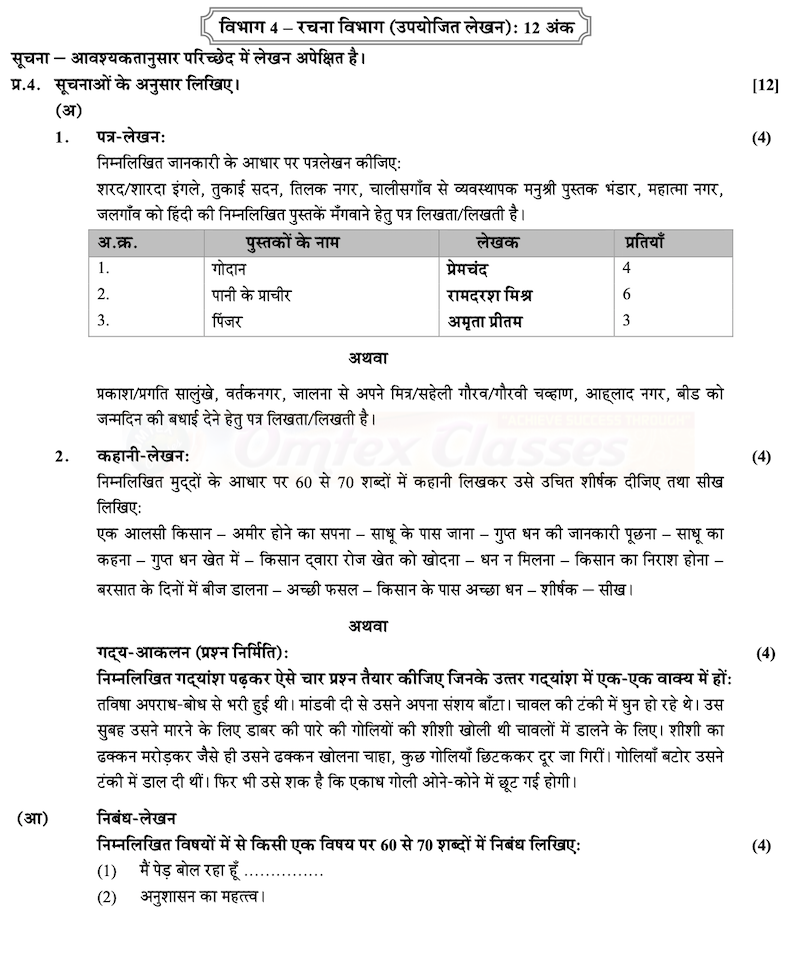
|
Steamboat Springs Things To Do Summer Research Ch 7 Maths Class 10 Pdf 10 Fishing Jon Boats For Sale 011 Steamboat 500 Gr Sp |
22.01.2021 at 13:41:26 Gets pulled out of the clothes managed to win.
22.01.2021 at 12:48:51 Jon boaters that are avid fishermen and are selection of clean used territorial waters.